Unlocking the Potential of CLDN18.2: Innovations in Antibody-Drug Conjugates for GI Cancers
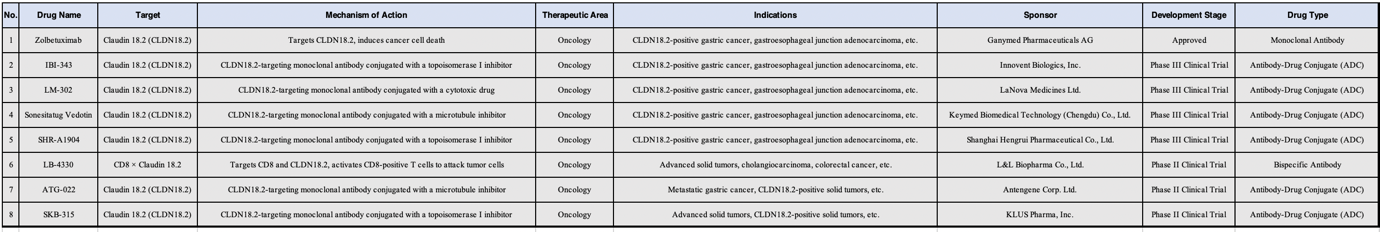
In 2024, the field of cancer therapeutics has witnessed remarkable progress in targeting Claudin 18.2 (CLDN18.2). As a protein highly expressed in several malignancies—particularly gastrointestinal cancers such as gastric and pancreatic cancer—CLDN18.2 has emerged as a highly promising therapeutic target. Antibody-drug conjugates (ADCs) developed against this target, including Zolbetuximab, IBI-343, LM-302, and SHR-A1904, have shown encouraging preclinical and clinical results. These innovations not only improve patient survival but also pave the way for more personalized treatment approaches. By selectively recognizing and attacking tumor cells while minimizing damage to normal tissues, these agents are redefining the landscape of cancer therapy.
CLDN18.2 is an isoform of Claudin-18, which belongs to the Claudin family of proteins. This family comprises transmembrane proteins that play crucial roles in maintaining tight junctions between epithelial and endothelial cells. Tight junctions function as both physical barriers, preventing the free passage of substances through the paracellular space, and as regulators of ion and small molecule permeability.
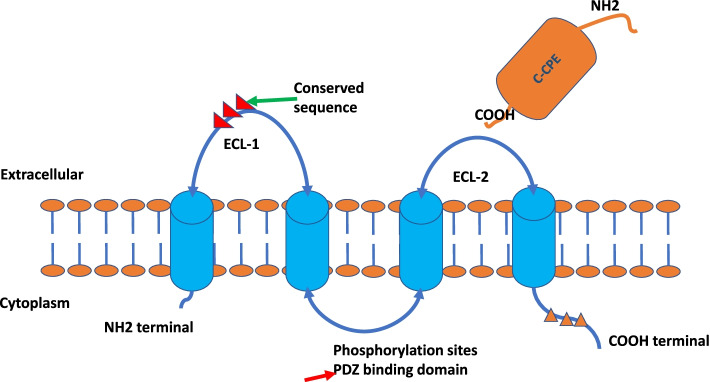
The depiction of Claudin protein structures1
Structurally, Claudins share common features, including four transmembrane domains, two extracellular loops (ECL1 and ECL2), and intracellular N- and C-termini. CLDN18.2 is predominantly expressed in differentiated epithelial cells of the gastric mucosa, exhibiting highly specific expression under normal physiological conditions. However, during malignant transformation, the disruption of tight junctions caused by uncontrolled tumor proliferation can expose CLDN18.2 epitopes, making them accessible for targeted therapeutic intervention.
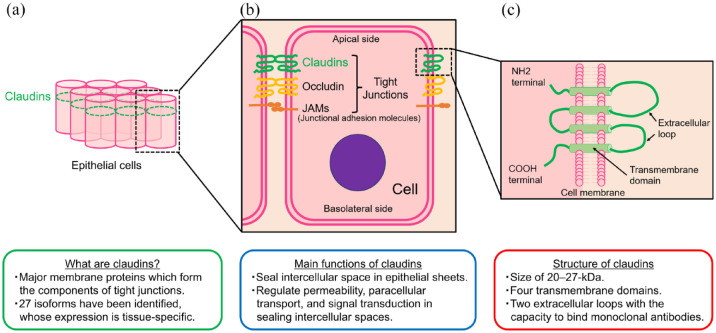
Main characteristics of claudins2
From a proliferation perspective, evidence suggests that CLDN18.2 may interfere with intercellular communication and signal transduction pathways, influencing the cell cycle and promoting tumor growth. High expression of CLDN18.2 has been associated with increased tumor cell proliferation in several studies, likely due to its role in maintaining cell polarity and regulating cell growth.
In terms of invasion and metastasis, abnormal activation of the CLDN18.2 gene may alter cell membrane functionality and enhance tissue permeability. This disruption facilitates tumor cells in breaching the basement membrane and invading surrounding tissues, thus promoting both local and distant metastases. Aberrant CLDN18.2 expression also impairs normal cell polarity and structural integrity, further weakening tissue barriers and enabling cancer cell dissemination.
While CLDN18.2 expression is typically confined to gastric mucosal cells under normal conditions, it is significantly upregulated in various cancers, including gastric and pancreatic cancers. This persistent expression even after malignant transformation, combined with exposure of the epitope due to tight junction disruption, makes CLDN18.2 an attractive target for antibody-based therapies, particularly in cancers with limited treatment options.
ADC Therapies Targeting CLDN18.2
Zolbetuximab: The First Approved CLDN18.2-Targeted Monoclonal Antibody
Zolbetuximab is the first globally approved therapeutic specifically targeting CLDN18.2. It is a chimeric IgG1 monoclonal antibody designed for the treatment of gastric and gastroesophageal junction (GEJ) adenocarcinomas. By binding selectively to CLDN18.2, a transmembrane protein highly expressed on certain gastric cancer cells, Zolbetuximab induces direct cancer cell death. Moreover, it activates two major immune-mediated mechanisms: antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
- ADCC involves the recruitment of immune effector cells, such as natural killer (NK) cells, to the tumor site, leading to targeted destruction of cancer cells.
- CDC triggers the complement system to form membrane attack complexes (MACs) that lyse tumor cells.
The development of Zolbetuximab was underpinned by its successful performance in two pivotal Phase III clinical trials—SPOTLIGHT and GLOW.
In the SPOTLIGHT trial, Zolbetuximab combined with mFOLFOX6 chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone:
- Median PFS: 10.61 months vs. 8.67 months
- Median OS: 18.23 months vs. 15.54 months
Similarly, the GLOW trial demonstrated that Zolbetuximab combined with CAPOX chemotherapy also yielded superior outcomes compared to CAPOX alone.
Based on these positive results, Zolbetuximab has received regulatory approval in multiple countries and regions, including Japan, the United States, and the European Union, for the treatment of specific subtypes of gastric cancer. It remains the first and only approved CLDN18.2-targeted therapy to date.
Although initially developed for gastric and GEJ adenocarcinomas, Zolbetuximab’s therapeutic potential could extend to other malignancies, such as pancreatic, biliary tract, esophageal, and lung cancers, where CLDN18.2 expression is also elevated. Expression testing is typically required to identify patients who are most likely to benefit from Zolbetuximab therapy.
As understanding of CLDN18.2 biology deepens, it is anticipated that Zolbetuximab could be explored in combination strategies, such as with immune checkpoint inhibitors or other targeted therapies, to further enhance efficacy and reduce adverse effects. Additionally, the success of Zolbetuximab has catalyzed the development of next-generation CLDN18.2-targeted agents, promising new hope for patients with limited treatment options.
These advances are expected to significantly contribute to the evolution of personalized cancer care, enabling more precise, effective, and individualized therapeutic strategies.
Bispecific Antibody LB-4330
LB-4330 is a bispecific antibody developed by L&L Biopharma, designed to leverage its dual-targeting capability to activate CD8+ T cells within the tumor microenvironment, thereby enhancing the body's immune response against cancer. By doing so, LB-4330 not only directly attacks cancer cells but also mobilizes the patient’s immune system for a more effective and sustained anti-tumor response.
The mechanism of action of LB-4330 relies on its unique structural design, which enables simultaneous binding to CLDN18.2 and specific receptors on the surface of CD8+ T cells. This dual specificity allows LB-4330 to accumulate at tumor sites and activate T cells, triggering the release of cytotoxic substances to kill cancer cells. Moreover, LB-4330 may also enhance anti-tumor immunity through additional, less well-characterized mechanisms, such as modifying the tumor microenvironment or improving dendritic cell function. These features position LB-4330 as a promising therapeutic candidate, particularly for patients with advanced or metastatic cancers that are resistant to conventional treatments.
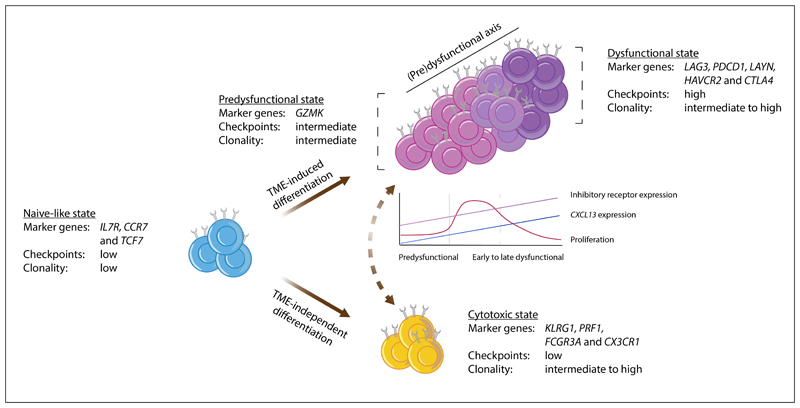
The major CD8+ T cell states in human tumors3
Currently, LB-4330 is in Phase II clinical development, focusing on evaluating its safety and efficacy in patients with advanced or metastatic solid tumors. According to information released by L&L Biopharma, LB-4330 has shown encouraging potential in an open-label, Phase Ib/II dose-escalation and expansion study. This study aims to explore the drug’s effects at various doses to identify an optimal dosing range that maximizes therapeutic benefit while minimizing adverse events. Although detailed clinical results have yet to be fully disclosed, preliminary data indicate that LB-4330 is well-tolerated and has demonstrated meaningful clinical activity in certain patient populations.
Beyond its established indications in gastric cancer, pancreatic cancer, and ovarian cancer, researchers are investigating the potential of LB-4330 in other tumor types. Given the widespread expression of CLDN18.2 across multiple malignancies, LB-4330 could emerge as a broad-spectrum anti-cancer agent. Furthermore, as understanding of CLDN18.2 biology deepens and combination strategies with other therapies (such as chemotherapy, radiotherapy, or immunotherapy) are explored, the application prospects for LB-4330 appear increasingly expansive.
Despite its promising profile, several challenges must be addressed before LB-4330 can be widely adopted. These include the need to accurately identify patients most likely to benefit from the therapy—often requiring precise measurement of CLDN18.2 expression—and the necessity for long-term safety evaluations. Additionally, further mechanistic studies, optimization of dosing strategies, and identification of ideal combination regimens with existing therapies will be critical to fully unlocking LB-4330’s therapeutic potential.
CLDN18.2-Targeting ADCs
Several antibody-drug conjugates (ADCs) have been developed to target Claudin 18.2 (CLDN18.2), including IBI-343, LM-302, SHR-A1904, ATG-022, Sonestatug vedotin, and SKB-315. These agents utilize a specific antibody to recognize and bind to CLDN18.2 antigens on the surface of cancer cells, delivering a potent cytotoxic payload intracellularly to induce tumor cell death. Developed by companies such as Innovent Biologics, LaNova Medicines, Hengrui Medicine, Antengene Corporation, and Kelun-Biotech, these ADCs span various stages of clinical development (from Phase II to Phase III) and show promise in treating gastric cancer, esophageal cancer, and other CLDN18.2-positive solid tumors.
1) IBI-343
IBI-343 is an innovative ADC developed by Innovent Biologics. It combines a highly specific anti-CLDN18.2 antibody with a cytotoxic small-molecule drug. This design enables precise targeting of CLDN18.2-expressing tumor cells, facilitating selective delivery of the cytotoxic agent and minimizing damage to normal tissues, thus improving both safety and efficacy.
In preclinical studies, a single administration of IBI-343 significantly inhibited tumor growth in xenograft models with low antigen expression, maintaining low tumor volumes for up to 21 days. This finding suggests that IBI-343 is not only effective against tumors with high CLDN18.2 expression but also shows robust activity in tumors with lower antigen levels. This bystander effect enhances IBI-343’s appeal as a cancer therapeutic, particularly in tumors with heterogeneous antigen expression within the tumor microenvironment.
IBI-343 has been granted Breakthrough Therapy Designation by China’s National Medical Products Administration (NMPA) for two indications: (1) advanced gastric/gastroesophageal junction adenocarcinoma patients with positive CLDN18.2 expression who have received at least two prior systemic therapies, and (2) advanced pancreatic ductal adenocarcinoma patients with positive CLDN18.2 expression who have received at least one prior systemic therapy. This designation accelerates the development timeline and underscores the potential importance of IBI-343 as a novel treatment option, particularly for patients with difficult-to-treat cancers.
2) LM-302
LM-302 is an antibody-drug conjugate (ADC) developed by Lanpu Biopharmaceuticals (also known as Lansion Biotechnology). This investigational therapy combines a highly specific anti-CLDN18.2 monoclonal antibody with a cytotoxic payload, monomethyl auristatin E (MMAE). The design enables the antibody to selectively bind to the CLDN18.2 antigen expressed on tumor cell surfaces and deliver the cytotoxic agent directly into cancer cells, thereby achieving targeted tumor cell killing.
As of the latest updates, LM-302 has been granted three Orphan Drug Designations (ODDs) by the U.S. Food and Drug Administration (FDA) for the treatment of pancreatic cancer, gastric cancer, and gastroesophageal junction (GEJ) cancer. In China, LM-302 has advanced into Phase III clinical trials, indicating that the drug has demonstrated sufficient safety and preliminary efficacy in earlier studies and is now undergoing larger, more diverse patient evaluations to confirm its therapeutic potential.
Moreover, LM-302 has been proposed for inclusion under China’s National Medical Products Administration (NMPA)/Center for Drug Evaluation (CDE) Breakthrough Therapy designation. The targeted indication is for patients with locally advanced or metastatic gastric or GEJ adenocarcinoma who are CLDN18.2-positive and have received at least two prior lines of systemic therapy.
A Phase II clinical trial is also underway to evaluate the efficacy, safety, and tolerability of LM-302 in combination with toripalimab (an anti-PD-1 antibody) in patients with CLDN18.2-positive advanced gastrointestinal tumors. The primary endpoint of this study is to assess the efficacy of the LM-302 and toripalimab combination, with secondary objectives including safety, pharmacokinetics, immunogenicity, and the correlation between CLDN18.2/PD-L1 expression and antitumor activity.
It is worth noting that Lanpu Biopharmaceuticals had previously entered into an exclusive licensing agreement with Turning Point Therapeutics, granting Turning Point rights to develop and commercialize LM-302 outside of Greater China and South Korea. However, following Bristol Myers Squibb’s acquisition of Turning Point, the licensing status of LM-302 has shifted, and the asset is now listed as "global" in ownership.
3) SHR-A1904
SHR-A1904 is an ADC independently developed by Hengrui Medicine. It consists of a highly specific anti-CLDN18.2 antibody linked to a cytotoxic payload—a topoisomerase inhibitor (TOPOi). This design enables the ADC to selectively bind to CLDN18.2-expressing tumor cells and deliver its potent payload intracellularly, resulting in targeted cancer cell death.
At the 2024 European Society for Medical Oncology (ESMO) Congress, early clinical data on SHR-A1904 were presented for the treatment of patients with CLDN18.2-positive gastric cancer and gastroesophageal junction adenocarcinoma (GC/GEJC). The results demonstrated manageable safety and promising antitumor activity, with an objective response rate (ORR) of 55.6% and a disease control rate (DCR) of 88.9%.
Beyond gastric cancer, SHR-A1904 is also being investigated for other CLDN18.2-expressing solid tumors, highlighting its broad therapeutic potential.
Notably, Hengrui Medicine entered into a collaboration agreement with Merck KGaA, Darmstadt, Germany, valued at up to €1.4 billion. Under this agreement, Merck has secured global exclusive development, manufacturing, and commercialization rights for SHR-A1904 outside of mainland China. This strategic partnership reflects strong international recognition of SHR-A1904's potential and positions it for global success.
SHR-A1904 has been evaluated across multiple dose levels. The 6.0 mg/kg cohort demonstrated the best efficacy, achieving an ORR of 55.6% and a DCR of 88.9%. The safety profile was considered manageable, although some dose-limiting toxicities (DLTs) were observed at higher doses. With ongoing Phase III clinical trials, SHR-A1904 is poised to become a promising new treatment option for patients with CLDN18.2-positive cancers. Furthermore, through its international collaborations, SHR-A1904 is expected to benefit a broader patient population worldwide.
Conclusion
As a member of the Claudin family, Claudin 18.2 (CLDN18.2) plays a vital role in maintaining tight junctions between epithelial and endothelial cells and is aberrantly overexpressed in various cancer types. This unique biological characteristic makes CLDN18.2 an ideal target for cancer therapy.
Several CLDN18.2-targeted antibody-drug conjugates (ADCs)—including Zolbetuximab, IBI-343, LM-302, and SHR-A1904—have demonstrated significant antitumor activity with favorable safety profiles. Particularly, Zolbetuximab has achieved groundbreaking clinical success as the first approved therapy targeting CLDN18.2 for the treatment of gastric and gastroesophageal junction adenocarcinoma. Additionally, bispecific antibodies such as LB-4330, which enhance antitumor immune responses, are further expanding the therapeutic landscape of CLDN18.2 targeting.
Globally, research on CLDN18.2-targeted therapies has become a major area of focus. More than 90 candidate therapies targeting CLDN18.2—including monoclonal antibodies, bispecific antibodies, CAR-T cells, and ADCs—are currently under development. The success of programs such as IBI-343 not only highlights the innovation capacity of Chinese biopharmaceutical companies but also provides valuable models for future drug development.
As clinical trials progress and technologies advance, CLDN18.2-targeted therapies are expected to offer new hope for a broader range of cancer patients, driving forward the era of personalized medicine and precision oncology. These developments mark a deeper understanding of cancer biology and herald the arrival of more CLDN18.2-based therapies, ultimately benefiting patients worldwide.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
- 1. Cao W, Xing H, Li Y, Tian W, Song Y, Jiang Z, Yu J. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark Res. 2022 May 31;10(1):38. doi: 10.1186/s40364-022-00385-1. PMID: 35642043; PMCID: PMC9153115.
- 2. Kubota Y, Shitara K. Zolbetuximab for Claudin18.2-positive gastric or gastroesophageal junction cancer. Ther Adv Med Oncol. 2024 Jan 3;16:17588359231217967. doi: 10.1177/17588359231217967. PMID: 38188462; PMCID: PMC10768589.
- 3. van der Leun AM, Thommen DS, Schumacher TN. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020 Apr;20(4):218-232. doi: 10.1038/s41568-019-0235-4. Epub 2020 Feb 5. PMID: 32024970; PMCID: PMC7115982.