Dobutamine Hydrochloride Market Analysis in the USA: Generic Landscape, Patents, and Clinical Insights
Overview
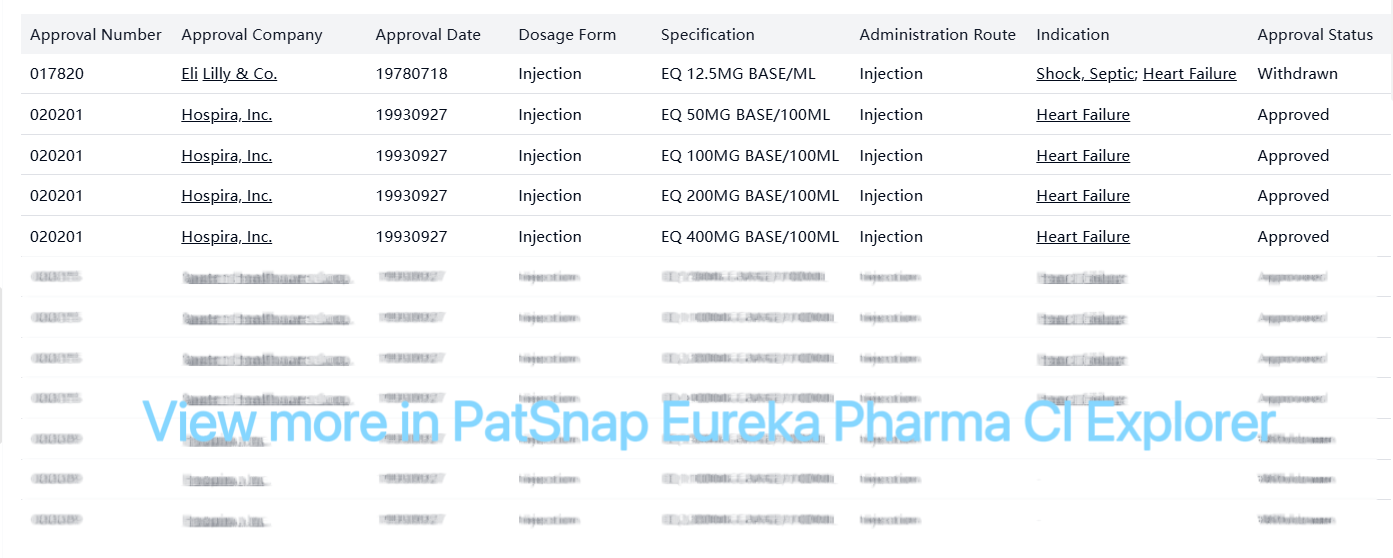
Dobutamine Hydrochloride is an established drug in the US market, with only 1 approved drug identified in our analysis. The drug was originally developed by Eli Lilly & Co. and is now marketed by multiple companies including Hospira, Inc. and Baxter Healthcare Corp. It is primarily used for heart failure treatment, administered as an injection with various strength formulations. As a generic drug with all original patents expired, it represents a stable market with established clinical use patterns and competitive generic presence.
Detailed Description
Drug Information
Dobutamine Hydrochloride was originally developed by Eli Lilly & Co. and is now approved in the USA.
Structure
Patent Barrier Analysis
Registration Patent Analysis
No registration patents were found in the FDA Orange Book for Dobutamine Hydrochloride.
Other Patent Barrier Analysis
Original Patents (All Expired)
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant | Country |
|---|---|---|---|---|---|---|
| US3987200A | Inactive | 19750115 | 19931019 | New Use | Eli Lilly & Co. | US |
| US4581225A | Inactive | 19850128 | 20040425 | New Use, Formulation | Eli Lilly & Co. | US |
| EP0187019A3 | Inactive | 19851218 | 20051218 | Drug Combination, Process, Formulation, Crystal Form | Eli Lilly & Co. | EP |
| EP0492930A2 | Inactive | 19911217 | - | Formulation | Eli Lilly & Co. | EP |
| CN1014774B | Inactive | 19851219 | 20051219 | New Use | Eli Lilly & Co. | CN |
| JP1987021343B2 | Inactive | 19820827 | 20020827 | - | Eli Lilly & Co. | JP |
Non-Original Patents (Selected Active Patents)
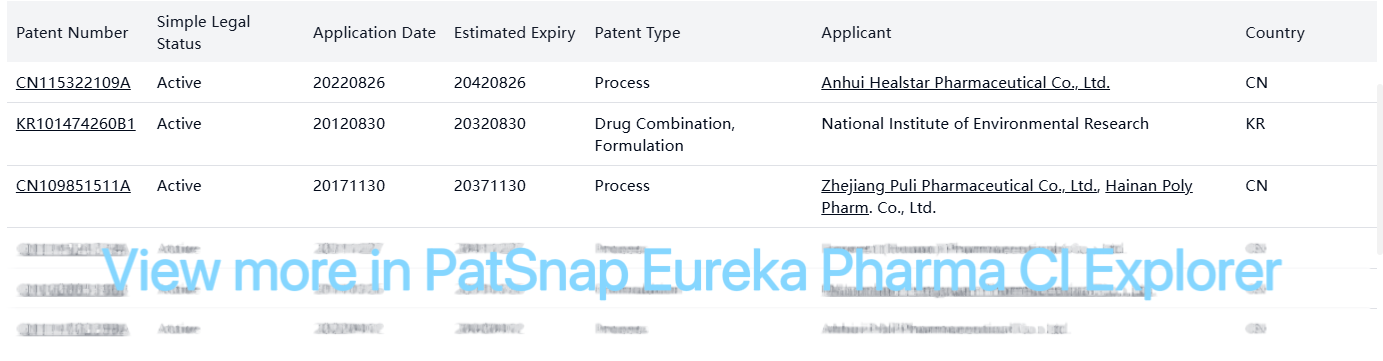
All original patents for Dobutamine Hydrochloride have expired. The active patents that exist are primarily process and formulation patents held by various companies, mostly in China and Korea. These patents do not present significant barriers to generic entry in the US market, as they are either jurisdiction-limited or relate to specific manufacturing processes rather than the compound itself.
Clinical Results
Preclinical Animal Studies
Early experiments compared dobutamine's hemodynamic effects with other adrenergic agents. For a given inotropic effect, dobutamine produced a smaller increase in heart rate and less pronounced decrease in peripheral vascular resistance than isoproterenol. These experiments elucidated the direct-acting nature of dobutamine on the β-receptors of the heart and provided a foundation for understanding its relative safety profile in terms of tachycardic and vasodilatory effects.
Pharmacodynamic and Pharmacokinetic Studies
Studies demonstrated that when administered in 5% dextrose injection, the onset of action occurs within 1 to 2 minutes, although up to 10 minutes might sometimes be needed to achieve the maximal effect. The plasma half-life of the drug was determined to be approximately 2 minutes. Metabolic pathway experiments identified the principal metabolic routes as methylation and conjugation, leading to inactive metabolites such as the 3-O-methyl derivative.
Controlled Clinical Trials and Short-Term Infusion Studies
Clinical experiments in patients with cardiac decompensation (resulting from depressed contractility due either to organic heart disease or following cardiac surgery) were executed using dobutamine in controlled trial settings. These studies evaluated the improvement in cardiac output and stroke volume, and while both dobutamine and other agents (such as isoproterenol) were found to increase output in patients with depressed cardiac function, dobutamine was observed to do so with less increase in heart rate and a more marked increase in stroke volume.
Hemodynamic Monitoring and Drug Interaction Studies
Studies underscored the importance of correcting hypovolemia prior to treatment and highlighted that dobutamine's effects could be altered in patients who had recently received β-blocking drugs. Experiments involving the co-administration of dobutamine with nitroprusside demonstrated that the combination could lead to a higher cardiac output and a lower pulmonary wedge pressure compared to when either agent was used alone.
Short-Term Versus Long-Term Treatment Comparisons
Controlled trials comparing short-term use versus chronic therapy consistently showed that long-term treatment did not result in improved symptoms and was instead associated with an increased risk of hospitalization and death. These findings supported the use of dobutamine only as a short-term inotropic support agent in a clinical setting.
Infringement Cases
Based on the provided reference, there is no information regarding any patent infringement incidents involving the drug Dobutamine Hydrochloride.
Policy and Regulatory Risk Warning
After a comprehensive search, Dobutamine Hydrochloride has no market exclusivity or data protection period in the USA. All original patents have expired, and there are no regulatory barriers to generic entry.
Market Entry Assessment & Recommendations
Generic Market Opportunity: With all original patents expired and no exclusivity periods in effect, Dobutamine Hydrochloride represents a stable generic market opportunity in the USA. Companies can enter this market with minimal patent barriers.
Manufacturing Process Innovation: While the compound itself is not patent-protected, there are active process patents in other jurisdictions. Companies entering the US market should develop non-infringing manufacturing processes or consider licensing arrangements with process patent holders if planning global production.
Formulation Differentiation: Consider developing improved formulations or delivery systems to differentiate from existing products. Several formulation patents exist globally that could provide inspiration for innovation while avoiding infringement.
Clinical Positioning: Focus marketing efforts on the established clinical benefits of dobutamine compared to other inotropic agents, particularly its favorable profile regarding heart rate and peripheral vascular resistance effects.
Supply Chain Optimization: As a mature generic product, cost efficiency and reliable supply will be key competitive factors. Develop robust supply chains and consider vertical integration to control costs.
Regulatory Strategy: Given the established safety profile and long history of use, consider abbreviated regulatory pathways that leverage existing data to expedite approval and market entry.
Market Focus: Target hospitals and acute care settings where dobutamine is primarily used for short-term inotropic support in heart failure and post-cardiac surgery patients.
Partnership Considerations: For companies new to injectable products, consider partnerships with established manufacturers to overcome technical and regulatory barriers to entry in this specialized dosage form.
For more scientific and detailed information of Dobutamine Hydrochloride , try PatSnap Eureka Pharma CI Explorer.