Request Demo
Pharmaceutical Insights
Navigate pharmaceutical trends with our insights on targets, institutional pipelines, clinical advances, and new drugs.

Recent blog posts
"What" Series
2 min read
What are the methods for radiolabeling antibodies?
23 April 2024
Commonly used radioactive isotopes include 125I and 131I, which can undergo iodination reactions with the tyrosine residues in the antibody.
Latest Hotspot
3 min read
GV20 Therapeutics Partners for Clinical Trial of New Immune Checkpoint Antibody GV20-0251 with KEYTRUDA®
23 April 2024
GV20 Therapeutics Reveals Partnership for Clinical Study to Test GV20-0251, an Innovative Antibody Targeting New Immune Checkpoint IGSF8, Together with KEYTRUDA® (pembrolizumab).
Feature Updates
4 min read
Utilize Synapse Database to Explore Translational Medicine!
23 April 2024
The Synapse Database for Translational Medicine is now live, come and experience it!
Latest Hotspot
3 min read
Phase 3 SELECT-GCA Study: Upadacitinib Shows Promise in Treating Giant Cell Arteritis
23 April 2024
Results from the Phase 3 SELECT-GCA trial indicate that Upadacitinib (RINVOQ®) offers promising outcomes for individuals with Giant Cell Arteritis.
"What" Series
2 min read
What are the requirements for the fragment length in the shRNA design process?
23 April 2024
In the design process of shRNA (short hairpin RNA), the fragment length is an important consideration factor, as it can affect the efficiency and specificity of RNA interference (RNAi).
Latest Hotspot
3 min read
NMPA Approves HLX53 Anti-TIGIT Trial with HANSIZHUANG/HANBEITAI for Advanced Liver Cancer
23 April 2024
NMPA Greenlights IND Submission for Initial Clinical Trial Using HLX53 Anti-TIGIT Fc Fusion Protein in Conjunction with HANSIZHUANG and HANBEITAI for Early Treatment in Patients with Advanced or Metastatic Liver Cancer.
Pharma Frontiers
10 min read
Pharma Frontiers: Daily Digest of Global Pharmaceutical News - April 23
23 April 2024
April 23rd latest updates in the global new drug development field, including progress in new drug research and development, transaction information, and partnership developments.
Latest Hotspot
3 min read
Vertex Reveals Progress in Suzetrigine (VX-548) for Acute and Neuropathic Pain Treatment
23 April 2024
Vertex Pharmaceuticals reports advancements in its suzetrigine pain treatment program. Suzetrigine, also known as VX-548, is an oral NaV1.8 inhibitor that could be the first new painkiller class for acute and neuropathic pain in two decades.
Graphic Pharma
11 min read
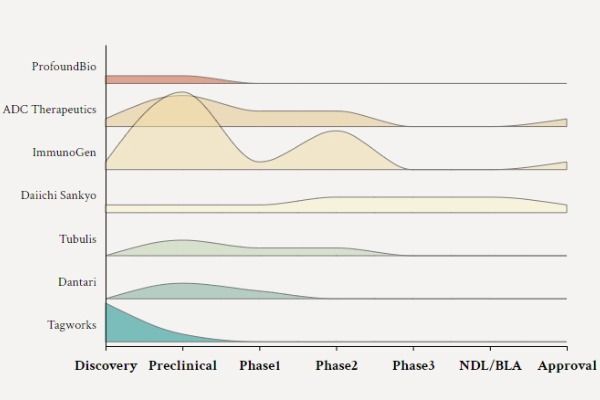
Summary of ADC Drug Development Trends! Revenue to Exceed 26 Billion US Dollars
22 April 2024
In the field of tumor treatment, antibody-drug conjugates (ADCs) have the potential to enhance the efficacy and specificity of cancer therapy by combining monoclonal antibodies with potent cytotoxic drugs.
Drug Highlight
14 min read
Global New Drug Research and Development Progress Weekly Report(4.15-4.21)
22 April 2024
4.15-4.21 Global Drug R&D Progress & Pharmaceutical Transaction Trends.
"What" Series
2 min read
What does interchangeability mean for biological drugs?
22 April 2024
Interchangeability in biosimilars means they match the original biological drug in quality, safety, and effectiveness, producing identical clinical outcomes.
Pharma Frontiers
10 min read
Pharma Frontiers: Daily Digest of Global Pharmaceutical News - April 20
22 April 2024
April 20th latest updates in the global new drug development field, including progress in new drug research and development, transaction information, and partnership developments.